Abstract
The new electric power systems based on new energy is a power system that is supported by source-grid-load-storage interaction and multi-energy complementarity, in order to reduce the carbon emission level of the new electric power systems, it is necessary to change the existing energy structure of the system through different energy carriers. Through technology comparison and analysis, seawater in-situ electrolysis hydrogen production technology can make full use of offshore renewable energy, which can effectively reduce the production cost of hydrogen production by electrolysis. With the development and industrial application of the third-generation low-energy phase change absorber, the technology of carbon dioxide capture by phase change solvent has great application prospects. The industrial application of carbon dioxide hydrogenation to methanol can rely on the existing mature C1 chemical system, and the new carbon dioxide hydrogenation to methanol device can improve the effective utilization rate of carbon dioxide and reduce the emission of tail gas. On this basis, the application scheme of carbon dioxide hydrogenation to methanol technology in the new power system is proposed: centered on the power grid, renewable energy is used to generate electricity, and part of the electricity is sent to the grid; part of the electricity is sent to the seawater in-situ electrolysis hydrogen production unit to produce hydrogen; the main product hydrogen is stored in the hydrogen storage equipment, and the by-product oxygen is used comprehensively; the carbon dioxide generated in the process of fossil fuel power generation is captured by phase change solvent, and then the new carbon dioxide hydrogenation to methanol device is used to synthesize carbon dioxide and hydrogen into methanol under the action of catalyst, part of the methanol is converted into hydrogen by cracking and stored, and the other part of methanol is directly used as the fuel of internal combustion power locomotives, when the power output of the grid is insufficient, methanol can be used as fuel for internal combustion generators, which are used to generate electricity and sent to the grid, and the stored hydrogen can also be used as fuel for hydrogen fuel cells, which are used to generate electricity and sent to the grid.
Keywords
Carbon Dioxide, Hydrogenation, Methanol, New Electric Power Systems, Application Prospect
1. Introduction
In order to promote the reduction of greenhouse gas emissions, mainly carbon dioxide, the Chinese government has proposed the following roadmap for carbon emission reduction: strive to achieve "carbon peak" before 2030 and "carbon neutrality" before 2060, and build a new electric power system based on new energy during the critical period and window period of "carbon peak" from 2021 to 2025. It is estimated that China's carbon emissions will reach a peak of 11.6 billion tons by 2030, of which 85.4% of the carbon is produced by energy activities.
New energy refers to various forms of energy other than traditional energy, such as solar energy, geothermal energy, wind energy, ocean energy, biomass energy and nuclear fusion energy. The new electric power systems, based on new energy sources, emphasize source-grid-load-storage interaction and multi-energy complementarity, they are characterized by being clean, low-carbon, safe, controllable, flexible, efficient, intelligent, friendly, and promoting open interaction. The intermittent and fluctuating nature of renewable energy sources like wind and solar power poses challenges to the traditional power system's efficiency, flexibility, security, and stability, the mismatch between renewable energy development and power consumption capacities requires new electric power systems to effectively manage complex energy demands.
As the most important greenhouse gas, carbon dioxide is the final product of hydrocarbon combustion, as a valuable carbon-containing resource, carbon dioxide can be effectively utilized through chemical methods. The use of carbon dioxide, hydrogen and methanol as chemical raw materials and clean fuels in the new electric power system can improve the comprehensive utilization efficiency of energy, addressing the challenge of renewable energy utilization, enhancing the flexibility of the new electric power system, and mitigating carbon emissions from the new electric power systems.
2. The Current State of China's Power System
By the conclusion of December 2023, China had amassed an installed power generation capacity of approximately 2919.65 GW, of which 1472.37 GW is renewable energy power generation, 1390.32 GW is thermal power generation, and 56.91 GW is nuclear power generation, and in 2023, China's installed renewable energy power generation capacity accounts for 50.43% of the total installed power generation capacity, and the installed capacity of renewable energy power generation exceeds the installed capacity of thermal power generation for the first time.
Table 1 shows the charting of China's 2023 renewable power generation capacity, of which solar power generation accounted for 41.40%, wind power accounted for 29.97%, and hydropower accounted for 28.63%, compared with 2022, the ranking of solar power generation has risen from second to first, the ranking of hydropower generation has dropped from first to third, and the ranking of wind power generation has risen from third to second. In 2023, China's new energy power generation capacity, including solar and wind power, reached 1050.83 GW, representing 36% of the total installed power generation capacity.
Table 1. List of China's Renewable Energy Power Generation Capacity in 2023.
Category | Power generation installed capacity/GW | Percentage of total installed power generation capacity/% |
Solar power | 609.49 | 41.40 |
Wind power | 441.34 | 29.97 |
Hydropower | 421.54 | 28.63 |
3. Electrolysis Hydrogen Production Technology
3.1. Hydrogen Production by Alkaline Electrolysis of Water
At present, hydrogen production by water electrolysis mainly includes three technical routes, namely alkaline electrolysis (ALK), proton exchange membrane electrolysis (PEM) and solid oxide electrolysis (SOEC), among which alkaline water electrolysis technology is relatively mature and has been widely used
| [1] | Wang Yuwei, Lu Haiyong, Sun Peifeng, et al. Research on the configuration and economy of new energy hydrogen production [J]. Electric Power and Energy, 2020, 41(5): 610-613, 631. |
[1]
.
Figure 1 is the schematic diagram of hydrogen production by alkaline electrolysis of water. Electric current is applied to the electrolytic cell, initiating an electrochemical reaction on the electrode where water molecules are broken down into hydrogen and oxygen. Hydrogen forms near the cathode, while oxygen is produced near the anode. Equation (
1), equation (
2), and equation (
3) represent the chemical reactions at the cathode, anode, and the overall reaction, respectively.
The electrolysis of water to produce hydrogen is a straightforward and environmentally friendly process, with production efficiency typically ranging from 75% to 85%. The electricity consumption per cubic meter of hydrogen is around 4 kWh to 5 kWh.
| [2] | Zhang Yunzhou, zhang Ning, Dai Hongcai, et al. Model construction and pathways of low-carbon transition of China's power system [J]. Electric Power, 2021, 54(3): 1-11. |
[2]
. The expenses involved in producing hydrogen through water electrolysis primarily consist of power costs and equipment costs. Power costs typically represent the largest portion, ranging from 40% to 80% of the total
| [3] | AHMAD K, UPADHYAYULA S. Greenhouse gas CO2 hydrogenation to fuels: a thermodynamic analysis [J]. Environmental Progress & Sustainable Energy, 2019, 38(1): 98-111. |
[3]
. The electrolyzer cost makes up approximately 40% to 50% of the equipment expenses, while auxiliary machinery accounts for about 50% to 60%.
| [4] | Cao Fan, Chen Kunyang, Guo Tingting, et al. Research on the technology path of hydrogen energy industry development [J]. Distributed Energy, 2020, 5(1): 1-8. |
[4]
. Currently, the main factor hindering the large-scale application of electrolysis hydrogen production technology is the high cost of electricity.
Figure 1. Diagram illustrating hydrogen generation through alkaline electrolysis of water.
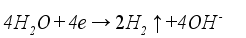 (1)
(1) 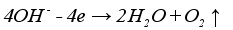 (2)
(2) 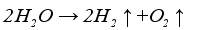 (3)
(3) 3.2. In-Situ Electrolysis of Seawater to Produce Hydrogen
Seawater contains a large number of impurities such as ions, microorganisms and particles, and the electrolysis of seawater to produce hydrogen will cause problems such as side reaction competition, catalyst inactivation, and diaphragm blockage. The on-site direct electrolysis method of seawater, without the need for desalination, combines water purification through a self-driven phase change mechanism with seawater electrolysis. This approach utilizes a hydrophobic porous polytetrafluoroethylene (PTFE) membrane as the gas pathway interface and employs concentrated potassium hydroxide (KOH) solution as a self-dampening electrolyte (SDE).
Figure 2 is a schematic diagram of in-situ electrolysis of seawater hydrogen production technology, this technique allows the diffusion of water vapor but completely prevents the penetration of liquid seawater and impurity ions. During operation, the disparity in water vapor pressure between the seawater side and the electrolyte side induces the seawater to evaporate naturally and diffuse as vapor across the membrane to the electrolyte side. Here, it undergoes re-liquefaction through absorption by the electrolyte. This phase change migration process enables the on-site production of pure water from seawater for electrolysis with 100% ion blocking efficiency. The water consumed by electrolysis within the electrolyte effectively sustains the pressure gradient at the interface. By maintaining a balance between the water migration rate and the electrolysis rate, a new thermodynamic equilibrium is established between the seawater and electrolyte. This results in a continuous and stable water transfer through a "liquid-gas-liquid" mechanism, ensuring a consistent supply of fresh water for electrolysis.
Figure 2. Schematic diagram of in-situ electrolysis of seawater for hydrogen production.
The on-site seawater electrolysis hydrogen production technology leverages affordable and lower-grade electricity sources like offshore wind power and solar energy. By integrating these with the renewable energy power generation system in remote ocean areas, an all-encompassing on-site electrolysis seawater hydrogen production system can be established, this integration effectively cuts down the production costs associated with seawater electrolysis for hydrogen production.
4. Carbon Capture Technology
4.1. Phase Change Solvents Capture Carbon Dioxide
Phase change solvent capture carbon dioxide technology referred to as PCS, phase change solvent capture carbon dioxide process flow is shown in
Figure 3, by using a phase dispersive solution consisting of 30% ethanolamine, 40% n-propanol, and 30% water, the pretreated flue gas undergoes reverse contact in the absorption tower to react and form carbamate. N-propanol serves as a physical solvent and phase change absorber, enhancing the gas-liquid mass transfer coefficient. Due to variances in hydrogen bonding forces, the hydrogen bonding between the product carbamate and water surpasses that between it and n-propanol. This leads to a competition for water molecules in the system, resulting in the discharge of n-propanol. The carbon dioxide-rich liquid coalesces into the lower aqueous phase, triggering liquid-liquid phase separation. The liquid-rich phase post-separation is conveyed by a liquid-rich pump, undergoing desorption of carbon dioxide in the desorption tower after heat exchange through the heat exchanger. Following desorption, the lean liquid from the tower bottom mixes with lean liquid (volume fraction: 41.2%) from the phase splitter and fresh absorbent addition, returning to the absorption tower for continued participation in the circulation absorption process. In the realm of fossil energy power generation, the technological and economic standpoint highlights significant application potential for phase change solvent carbon capture technology with the advancement and industrial implementation of third-generation low-energy phase change absorbers.
Figure 3. Process flow chart of phase change solvent capturing carbon dioxide.
4.2. Other Carbon Capture Technologies
Monoethanolamine carbon dioxide capture technology referred to as MEA, ethanolamine is commonly used as acid gas such as carbon dioxide absorbent, has the characteristics of fast absorption rate, low price, etc., widely used in the power industry in the process of carbon dioxide absorption. The whole process mainly includes carbon dioxide absorption process and carbon dioxide desorption process. In the absorption process, two types of reactions take place, the first involves the reaction of ethanolamine with carbon dioxide to produce carbamate, the second type is the protonation reaction, where dissolved carbon dioxide in water dissociates into HCO3-, CO32-, and H3O+, subsequently, ethanolamine reacts with H3O+ to create the protonated salt MEAH.
Dimethyl carbonate carbon dioxide capture technology, commonly known as DMC, involves the flue gas entering the absorption tower from the bottom and coming into reverse contact with dimethyl carbonate solution sprayed from the top of the tower. The carbon dioxide-enriched liquid after absorption proceeds to the high-low pressure flash tower, where it simultaneously desorbs carbon dioxide gas. Post high-pressure and low-pressure flash stages, the lean liquid flows into the gas stripping column. In the gas stripping column, nitrogen is utilized as a gas stripping agent under normal pressure to further decrease the residual carbon dioxide content in the lean liquid. The tail gas discharged from the gas stripping column is directly released. The lean liquid is withdrawn from the bottom of the stripper tower and adjusted to the required temperature via the heat exchanger. Subsequently, the lean liquid is blended with supplementary dimethyl carbonate solution before being returned to the absorption tower for continued participation in the cyclic absorption process.
Gas membrane separation technology for carbon dioxide is commonly known as GMS, and it involves a process encompassing compression condensation and two-stage membrane separation. Initially, the compressed flue gas is directed to the condenser before being transferred to a gas-liquid separator post-condensation. Here, a majority of the water is separated, and the remaining portion progresses to the membrane for gas permeation, in the presence of a pressure gradient across the membrane, carbon dioxide within the flue gas can permeate through, while nitrogen-rich gas gathers at the outlet on the hold-up side. Ultimately, high-purity carbon dioxide is obtained at the outlet on the permeation side.
| [5] | Guo changqing, Yi liqi, Yan changfeng, et al. Optimization of solar photovoltaic-PEM water electrolysis direct coupling system for hydrogen production [J]. Progress in New Energy, 2019, 7(3): 287-294. |
[5]
.
5. Carbon Dioxide Hydrogenation to Methanol Technology
5.1. Introduction to the Technology
Methanol, also known as hydroxy methane, is an organic compound that is the simplest saturated monoalcohol. Methanol is a colorless transparent liquid with a pungent odor, the molecular formula is CH
3OH, the molecular weight is 32.04, and the CAS number is 67-56-1. The reaction process of carbon dioxide hydrogenation to methanol is simple, the process is becoming more and more mature, carbon dioxide and hydrogen are adsorbed on the surface of the polyatomic metal cluster catalyst, and gradually converted into gaseous methanol, of which the catalyst used is mostly Cu-Zn-Al system
| [6] | Guo Hao, Yang Honghai. Current status and future prospect of research on solid-state hydrogen storage material [J]. New Chemical Materials, 2016, 44(9): 19-21. |
[6]
.
The industrial application of carbon dioxide hydrogenation to methanol can rely on China's existing and mature C1 chemical system. At present, China's annual methanol production capacity is about 93 million tons, but most of the methanol is still synthesized from fossil fuels. The production of 1 ton of methanol in coal-to-methanol plants requires the emission of 3.5 tons-4.0 tons of carbon dioxide, among which the main sources of carbon dioxide emissions are the high energy consumption devices used in the air separation process of oxygen and the water-to-gas conversion units that adjust the ratio of carbon monoxide and hydrogen. Due to the large amount of carbon dioxide produced and emitted in the conversion of methanol from fossil fuels, China has banned the construction of coal-to-methanol production units with an annual capacity of less than 1 million tons.
Carbon dioxide and hydrogen in the catalyst under the action of the reaction to generate methanol, this reaction process can be represented by chemical equation (
4), in this reaction process, carbon dioxide and hydrogen are first adsorbed by the catalyst, and then chemical reaction to generate methanol and water, this reaction process needs to be carried out under high temperature and high-pressure conditions, usually need to use high temperature and high-pressure reactor
| [7] | Nie Congying, Shen Xiaojun, Lu Hong, et al. Capacity configuration and control strategy of hydrogen super hybrid energy storage in grid connected wind farm [J]. Smart Power, 2020, 48(9): 1-8. |
[7]
.
CO2 + 3H2 → CH3OH + H2O (4)
In the reaction system of carbon dioxide hydrogenation to methanol, the main chemical reactions are shown in Equation (
5), Equation (
6) and Equation (
7), where Equation (
5) and Equation (
6) are carbon dioxide hydrogenation reaction and carbon monoxide hydrogenation reaction, respectively, both of which belong to exothermic reactions and are entropy reduction reaction; Equation (
7) is the reverse water vapor transformation reaction, which is the endothermic reaction
| [8] | Li Haibo, Pan Zhiming, Huang Yaowen. Analysis on the application prospect of hydrogen fuel gas turbine power generation [J]. Electric Power Equipment Management, 2020(8): 94-96. |
[8]
.
CO2 + 3H2 ⇆ CH3OH + H2O (5)
Kaisar Ahmad et al. analyzed the effects of reaction pressure, reaction temperature and raw material composition on the hydrogenation of carbon dioxide to methanol by using a thermodynamic model. The results showed that the conversion rate of carbon dioxide increased with the increase of pressure, and showed a trend of first decreasing and then increasing with the increase of temperature
| [9] | Guo Mengjie, Yan Zheng, Zhou Yun, et al. Optimal operation of integrated energy system with wind power hydrogen production device [J]. China Electric Power, 2020, 53(1): 115-123, 161. |
[9]
. Increasing the pressure and decreasing the temperature are conducive to improving the selectivity of methanol, the conversion rate of carbon dioxide and the selectivity of methanol, and the selectivity of carbon monoxide increases with the decrease of pressure and the increase of temperature
| [10] | SHI C F, ZHANG T, LI J, et al. Powering the future with liquid sunshine [J]. Joule, 2018, 2(10): 1925-1949. |
[10]
. Under high-pressure and low-temperature reaction conditions, the synthesis of methanol from carbon dioxide is enhanced. However, due to thermodynamic equilibrium constraints, the unidirectional conversion rate of carbon dioxide and methanol productivity remain relatively low. Specifically, at 523K and 4MPa, the equilibrium conversion rate of carbon dioxide and methanol productivity are approximately 23% and 14%.
| [11] | Lu Yifei, Chen Chong, Liang Lizhong. Modeling and control of wind-hydrogen coupling system based on electricity-hydrogen hybrid energy storage [J]. Smart Power, 2020, 48(3): 7-14. |
[11]
. Hence, to boost the overall conversion of carbon dioxide, it is typically essential to implement a multi-step process or an exhaust gas recirculation process.
Carbon dioxide hydrogenation to methanol mainly includes direct hydrogenation, photocatalytic reduction, electrocatalytic reduction and biocatalytic reduction, etc. At present, direct hydrogenation has entered the industrial test link. Direct hydrogenation process directly from carbon dioxide and hydrogen as raw materials, through compression, synthesis, gas separation, rectification and other units to produce methanol.
Figure 4 is the process flow chart of carbon dioxide hydrogenation to methanol based on direct hydrogenation method. Following multiple stages of compression, carbon dioxide is blended with the recirculating gas comprising hydrogen and methanol, this mixture is then passed through a heat exchanger to attain the necessary reaction temperature before being fed into the methanol synthesis reactor.
| [12] | Li Jianqiang, Yu Guangzheng, Tang Bo, et al. Multi-energy flow integrated energy system planning considering wind and solar utilization and containing hydrogen energy flow [J]. Power System Protection and Control, 2021, 49(14): 11-20. |
[12]
.
Figure 4. Process flow chart of carbon dioxide hydrogenation to methanol based on direct hydrogenation method.
Figure 5. Schematic diagram of the structure of a carbon dioxide hydrogenation to methanol plant.
Due to the low initial conversion rate of carbon dioxide hydrogenation to methanol, the effluent from the reactor comprises a significant amount of unreacted carbon dioxide, hydrogen, and carbon monoxide alongside methanol. To address this, the effluent is condensed to 30°C and then subjected to high and low-pressure separation in a tank. The gas phase is split into two streams, the majority is recycled back to the methanol synthesis reactor, while a minor portion is discharged as tail gas, the liquid phase is directed to a methanol distillation column for purification, the crude methanol from the bottom of the gas-liquid separator is preheated to its bubble point temperature before entering the distillation column. Due to the relatively simple composition of the crude methanol produced by the carbon dioxide hydrogenation process, a single-column distillation approach with 33 plates, a reflux ratio of 2.3, and a pressure drop selection of 0.0068 atm is employed, a small quantity of carbon dioxide present in the liquid-phase crude methanol enters the methanol rectification tower. Non-condensable components such as carbon dioxide are vaporized from the tower's top, after compression and condensation, the crude methanol is separated in a flash tank, with the non-condensable gases removed from the top and purified methanol collected from the bottom, the final methanol recovery rate achieves 99.5wt.% with a mass fraction of 99.9 wt.%.
5.4. New Type of Equipment
At present, the equipment manufacturing of carbon dioxide hydrogenation to methanol has not yet achieved large-scale in China, mainly based on small-scale demonstration projects. In 2020, China developed a new carbon dioxide hydrogenation to methanol device, and
Figure 5 shows the schematic structure of the device, this new carbon dioxide hydrogenation to methanol device comprises a mixer, a reactor, a cooler, a flash tank and a tail gas diverter. The inner part of the reactor is filled with a catalyst bed. The inlet of the mixer is divided into three parts, and the two inlet of the mixer are respectively fed carbon dioxide and hydrogen
| [13] | LIN H Z, PEI A G, FANG M X. Progress of research on process modifications for amine solvent-based post combustion CO2 capture from coal-fired power plant [J]. Chemical Industry and Engineering Progress, 2018, 37(12): 4874-4886. |
[13]
. The outlet of the mixer is connected with the inlet of the reactor, and the outlet of the reactor is connected with the flash tank through the cooler.
| [14] | Han Shuqi, Li Wenxin, Chen Chong, et al. Modeling and control of controllable direct-drive permanent magnet wind turbine based on wind power hydrogen production and super capacitor hybrid energy storage [J]. Guangdong Electric Power, 2019, 32(5): 1-12. |
[14].
After cooling, the mixed gas discharged from the reactor enters the flash tank for gas-liquid separation, and the methanol is discharged from the outlet at the bottom of the flash tank. The outlet at the top of the flash tank is connected with the inlet of the tail gas diverter, and the outlet of the tail gas diverter is divided into two ways, one is discharged to the exhaust gas treatment system, and the other is connected to the third inlet of the mixer, so that a part of the tail gas is separated as a circulating gas into the next cycle. The inlet of the mixer is also connected to a carbon monoxide intake pipe for filling the mixer with fresh carbon monoxide
| [15] | Zhang Li, Chen Shuoyi. Development status and countermeasures of wind power hydrogen production technology at home and abroad [J]. Science and Technology China, 2020(1): 13-16. |
[15]
. The preheater is arranged on the pipe road between the outlet of the mixer and the inlet of the reactor, and the heat exchanger is arranged on the pipe road between the outlet of the reactor and the cooler; The media fluid is passed into the cold channel of the heat exchanger, and the mixed gas after the reaction is completed passes through the hot channel of the heat exchanger to transfer part of the heat in the mixed gas to the media fluid, and the heated media fluid is then used as the heat source of the preheater to heat the preheater
| [16] | Jiang Kangle. Research and environmental benefit evaluation of wind-solar hybrid hydrogen production system [D]. Handan: Hebei University of Engineering, 2018. |
[16]
.
After testing, compared with other similar devices, this new carbon dioxide hydrogenation to methanol plant can greatly improve the effective utilization rate of carbon dioxide (CO
2 conversion rate>60%) and reduce tail gas emissions
| [17] | Xu Jing, Zhao Xia, Luo Yinghong. Improved virtual synchronous generator control for hydrogen fuel cell integration into a microgrid [J]. Power System Protection and Control, 2020, 48(22): 165-172. |
[17]
.
6. Application Scheme of Methanol in New Electric Power System
6.1. Content of the Scheme
Hydrogen energy is a clean and zero-carbon secondary energy source, which has the characteristics of high energy density, diverse acquisition methods, clean production and use processes, and diverse application scenarios. Using hydrogen energy as the energy carrier in the power system, and converting hydrogen energy into electricity, heat energy and chemical energy for comprehensive utilization, can change the existing energy structure of the power system.
Figure 6. Schematic diagram of the application scheme of carbon dioxide hydrogenation to methanol technology in a new electric power system.
Figure 6 depicts a schematic illustration of the implementation plan for utilizing carbon dioxide hydrogenation to methanol technology in a novel electric power system. Taking the power grid as the center, using renewable energy power generation or valley electric in the power grid and directly electrolyzing seawater on the spot to produce hydrogen, the primary product, hydrogen, is stored in hydrogen storage units, while the by-product oxygen is effectively utilized.; the carbon dioxide generated in the process of fossil fuel power generation is captured by phase change solvent, and then the new carbon dioxide hydrogenation to methanol device is used to synthesize carbon dioxide and hydrogen into methanol under the action of catalyst, part of the methanol is converted into hydrogen by cracking and stored, and the other part of methanol is directly used as the fuel of internal combustion power locomotives, when the power output of the grid is insufficient, methanol can be used as fuel for internal combustion generators, which are used to generate electricity and sent to the grid, and the stored hydrogen can also be used as fuel for hydrogen fuel cells, which are used to generate electricity and sent to the grid.
6.2. Characteristics of the Scheme
Methanol is a low-carbon and oxygenated fuel, which has the characteristics of efficient combustion, clean emissions, and green renewable. Compared with hydrogen, methanol has a high energy density and is convenient to transport, and methanol can release hydrogen through cracking, so methanol is a good hydrogen storage carrier. Methanol can also be used as a liquid fuel for direct methanol fuel cells (DMFC) and modified diesel engines. The use of excess, off-peak or low-grade renewable energy to generate electricity, the use of seawater in-situ electrolysis hydrogen production technology, and the on-site production of hydrogen can effectively reduce the electricity cost of hydrogen. Using carbon dioxide hydrogenation to make methanol, renewable energy and carbon resources are stored in methanol in the form of chemical energy, and methanol, a liquid fuel, can be easily stored and transported. Methanol as a fuel for diesel locomotives can replace gasoline and diesel, the supplementary power generation of hydrogen fuel cell and internal combustion generator plays a role of peak cutting and valley filling.
By implementing carbon dioxide hydrogenation to methanol technology within the power system, carbon dioxide, hydrogen, and methanol serve as energy carriers to establish an energy network, thereby reshaping the existing energy landscape of the power system. Through the mutual conversion and utilization of renewable energy-electric energy- hydrogen energy-electrical energy-chemical energy, the energy utilization efficiency of the power system can be improved and the consumption problem of renewable energy in the system can be solved, this approach enhances the power system's flexibility and reduces carbon emissions, contributing to a more sustainable energy framework.
7. Conclusion
Hydrogen production by alkaline water electrolysis is a mature technology, but the high cost of electricity restricts the large-scale application of the technology. In situ electrolysis of seawater hydrogen production technology can make full use of low-cost offshore renewable energy, thus, it can significantly decrease the production expenses associated with electrolytic hydrogen generation. From a technical and economic point of view, with the development and industrial application of the third-generation low-energy phase change absorber, the technology of carbon dioxide capture by phase change solvent has great application prospects.
The industrial application of carbon dioxide hydrogenation to methanol can rely on China's existing mature C1 chemical system, and the recently developed new carbon dioxide hydrogenation to methanol plant can greatly improve the effective utilization rate of carbon dioxide and reduce tail gas emissions.
Taking the power grid as the center, renewable energy is used to generate electricity, and part of the electricity is sent to the grid; part of the electricity is sent to the seawater in-situ electrolysis hydrogen production unit to produce hydrogen; the main product hydrogen is stored in the hydrogen storage equipment, and the by-product oxygen is used comprehensively. Additionally, the carbon dioxide produced during fossil fuel power generation is captured using phase change solvents, and then the new carbon dioxide hydrogenation to methanol device is used to synthesize carbon dioxide and hydrogen into methanol under the action of catalyst, part of the methanol is converted into hydrogen by cracking and stored, and the other part of methanol is directly used as the fuel of internal combustion power locomotives, when the power output of the grid is insufficient, methanol can be used as fuel for internal combustion generators, which are used to generate electricity and sent to the grid, and the stored hydrogen can also be used as fuel for hydrogen fuel cells, which are used to generate electricity and sent to the grid.
Abbreviations
ALK: alkaline elec-trolysis
PEM: proton exchange membrane electrolysis
SOEC: solid oxide electrolysis
PTFE: hydrophobic porous polytetrafluoroethylene
SDE: self-dampening electrolyte
DMFC: direct methanol fuel cells
C1: refers to the process of converting and synthesizing chemical products or liquid fuels using a compound of one carbon atom as a raw material.
Acknowledgments
In the era of "carbon peak" and "carbon neutrality", CHINA ENERGY INVESTMENT is committed to green and low-carbon development. Here, I would like to express my deep respect to CHINA ENERGY INVESTMENT that nurtured our growth.
Author Contributions
Xin Nie is the sole author. The author read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
| [1] |
Wang Yuwei, Lu Haiyong, Sun Peifeng, et al. Research on the configuration and economy of new energy hydrogen production [J]. Electric Power and Energy, 2020, 41(5): 610-613, 631.
|
| [2] |
Zhang Yunzhou, zhang Ning, Dai Hongcai, et al. Model construction and pathways of low-carbon transition of China's power system [J]. Electric Power, 2021, 54(3): 1-11.
|
| [3] |
AHMAD K, UPADHYAYULA S. Greenhouse gas CO2 hydrogenation to fuels: a thermodynamic analysis [J]. Environmental Progress & Sustainable Energy, 2019, 38(1): 98-111.
|
| [4] |
Cao Fan, Chen Kunyang, Guo Tingting, et al. Research on the technology path of hydrogen energy industry development [J]. Distributed Energy, 2020, 5(1): 1-8.
|
| [5] |
Guo changqing, Yi liqi, Yan changfeng, et al. Optimization of solar photovoltaic-PEM water electrolysis direct coupling system for hydrogen production [J]. Progress in New Energy, 2019, 7(3): 287-294.
|
| [6] |
Guo Hao, Yang Honghai. Current status and future prospect of research on solid-state hydrogen storage material [J]. New Chemical Materials, 2016, 44(9): 19-21.
|
| [7] |
Nie Congying, Shen Xiaojun, Lu Hong, et al. Capacity configuration and control strategy of hydrogen super hybrid energy storage in grid connected wind farm [J]. Smart Power, 2020, 48(9): 1-8.
|
| [8] |
Li Haibo, Pan Zhiming, Huang Yaowen. Analysis on the application prospect of hydrogen fuel gas turbine power generation [J]. Electric Power Equipment Management, 2020(8): 94-96.
|
| [9] |
Guo Mengjie, Yan Zheng, Zhou Yun, et al. Optimal operation of integrated energy system with wind power hydrogen production device [J]. China Electric Power, 2020, 53(1): 115-123, 161.
|
| [10] |
SHI C F, ZHANG T, LI J, et al. Powering the future with liquid sunshine [J]. Joule, 2018, 2(10): 1925-1949.
|
| [11] |
Lu Yifei, Chen Chong, Liang Lizhong. Modeling and control of wind-hydrogen coupling system based on electricity-hydrogen hybrid energy storage [J]. Smart Power, 2020, 48(3): 7-14.
|
| [12] |
Li Jianqiang, Yu Guangzheng, Tang Bo, et al. Multi-energy flow integrated energy system planning considering wind and solar utilization and containing hydrogen energy flow [J]. Power System Protection and Control, 2021, 49(14): 11-20.
|
| [13] |
LIN H Z, PEI A G, FANG M X. Progress of research on process modifications for amine solvent-based post combustion CO2 capture from coal-fired power plant [J]. Chemical Industry and Engineering Progress, 2018, 37(12): 4874-4886.
|
| [14] |
Han Shuqi, Li Wenxin, Chen Chong, et al. Modeling and control of controllable direct-drive permanent magnet wind turbine based on wind power hydrogen production and super capacitor hybrid energy storage [J]. Guangdong Electric Power, 2019, 32(5): 1-12.
|
| [15] |
Zhang Li, Chen Shuoyi. Development status and countermeasures of wind power hydrogen production technology at home and abroad [J]. Science and Technology China, 2020(1): 13-16.
|
| [16] |
Jiang Kangle. Research and environmental benefit evaluation of wind-solar hybrid hydrogen production system [D]. Handan: Hebei University of Engineering, 2018.
|
| [17] |
Xu Jing, Zhao Xia, Luo Yinghong. Improved virtual synchronous generator control for hydrogen fuel cell integration into a microgrid [J]. Power System Protection and Control, 2020, 48(22): 165-172.
|
Cite This Article
-
-
@article{10.11648/j.ajee.20241201.13,
author = {Xin Nie},
title = {Application Prospect of Carbon Dioxide Hydrogenation to Methanol Technology in the New Electric Power Systems
},
journal = {American Journal of Energy Engineering},
volume = {12},
number = {1},
pages = {17-25},
doi = {10.11648/j.ajee.20241201.13},
url = {https://doi.org/10.11648/j.ajee.20241201.13},
eprint = {https://article.sciencepublishinggroup.com/pdf/10.11648.j.ajee.20241201.13},
abstract = {The new electric power systems based on new energy is a power system that is supported by source-grid-load-storage interaction and multi-energy complementarity, in order to reduce the carbon emission level of the new electric power systems, it is necessary to change the existing energy structure of the system through different energy carriers. Through technology comparison and analysis, seawater in-situ electrolysis hydrogen production technology can make full use of offshore renewable energy, which can effectively reduce the production cost of hydrogen production by electrolysis. With the development and industrial application of the third-generation low-energy phase change absorber, the technology of carbon dioxide capture by phase change solvent has great application prospects. The industrial application of carbon dioxide hydrogenation to methanol can rely on the existing mature C1 chemical system, and the new carbon dioxide hydrogenation to methanol device can improve the effective utilization rate of carbon dioxide and reduce the emission of tail gas. On this basis, the application scheme of carbon dioxide hydrogenation to methanol technology in the new power system is proposed: centered on the power grid, renewable energy is used to generate electricity, and part of the electricity is sent to the grid; part of the electricity is sent to the seawater in-situ electrolysis hydrogen production unit to produce hydrogen; the main product hydrogen is stored in the hydrogen storage equipment, and the by-product oxygen is used comprehensively; the carbon dioxide generated in the process of fossil fuel power generation is captured by phase change solvent, and then the new carbon dioxide hydrogenation to methanol device is used to synthesize carbon dioxide and hydrogen into methanol under the action of catalyst, part of the methanol is converted into hydrogen by cracking and stored, and the other part of methanol is directly used as the fuel of internal combustion power locomotives, when the power output of the grid is insufficient, methanol can be used as fuel for internal combustion generators, which are used to generate electricity and sent to the grid, and the stored hydrogen can also be used as fuel for hydrogen fuel cells, which are used to generate electricity and sent to the grid.
},
year = {2024}
}
 Copy
|
Copy
|
 Download
Download
-
TY - JOUR
T1 - Application Prospect of Carbon Dioxide Hydrogenation to Methanol Technology in the New Electric Power Systems
AU - Xin Nie
Y1 - 2024/04/02
PY - 2024
N1 - https://doi.org/10.11648/j.ajee.20241201.13
DO - 10.11648/j.ajee.20241201.13
T2 - American Journal of Energy Engineering
JF - American Journal of Energy Engineering
JO - American Journal of Energy Engineering
SP - 17
EP - 25
PB - Science Publishing Group
SN - 2329-163X
UR - https://doi.org/10.11648/j.ajee.20241201.13
AB - The new electric power systems based on new energy is a power system that is supported by source-grid-load-storage interaction and multi-energy complementarity, in order to reduce the carbon emission level of the new electric power systems, it is necessary to change the existing energy structure of the system through different energy carriers. Through technology comparison and analysis, seawater in-situ electrolysis hydrogen production technology can make full use of offshore renewable energy, which can effectively reduce the production cost of hydrogen production by electrolysis. With the development and industrial application of the third-generation low-energy phase change absorber, the technology of carbon dioxide capture by phase change solvent has great application prospects. The industrial application of carbon dioxide hydrogenation to methanol can rely on the existing mature C1 chemical system, and the new carbon dioxide hydrogenation to methanol device can improve the effective utilization rate of carbon dioxide and reduce the emission of tail gas. On this basis, the application scheme of carbon dioxide hydrogenation to methanol technology in the new power system is proposed: centered on the power grid, renewable energy is used to generate electricity, and part of the electricity is sent to the grid; part of the electricity is sent to the seawater in-situ electrolysis hydrogen production unit to produce hydrogen; the main product hydrogen is stored in the hydrogen storage equipment, and the by-product oxygen is used comprehensively; the carbon dioxide generated in the process of fossil fuel power generation is captured by phase change solvent, and then the new carbon dioxide hydrogenation to methanol device is used to synthesize carbon dioxide and hydrogen into methanol under the action of catalyst, part of the methanol is converted into hydrogen by cracking and stored, and the other part of methanol is directly used as the fuel of internal combustion power locomotives, when the power output of the grid is insufficient, methanol can be used as fuel for internal combustion generators, which are used to generate electricity and sent to the grid, and the stored hydrogen can also be used as fuel for hydrogen fuel cells, which are used to generate electricity and sent to the grid.
VL - 12
IS - 1
ER -
 Copy
|
Copy
|
 Download
Download
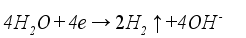 (1)
(1) 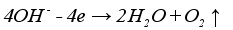 (2)
(2) 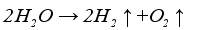 (3)
(3)